Partners & Medical Professionals
A wide body of evidence substantiates the efficacy of InsuJet™. Take a deeper dive into the research that speaks to its benefits.
Get started with the needle-free insulin injection system.
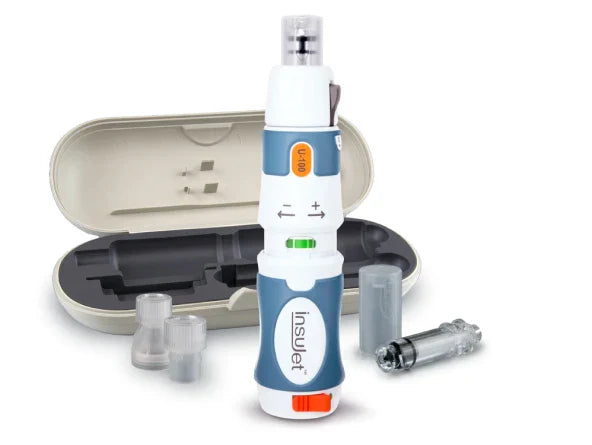
A wide body of evidence substantiates the efficacy of InsuJet™. Take a deeper dive into the research that speaks to its benefits.

